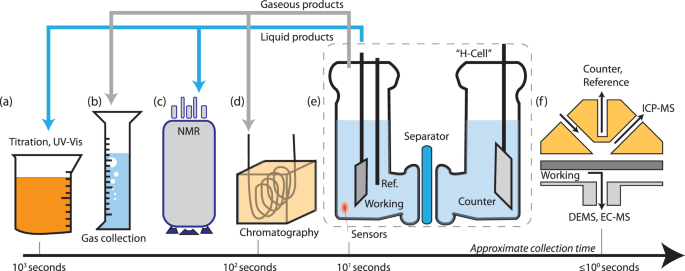
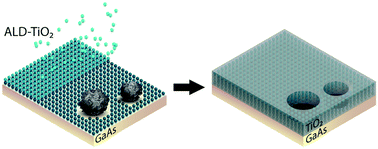
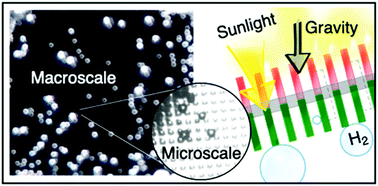
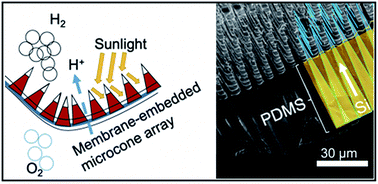
Our group researches physical (electro)chemical processes at interfaces used for grid-scale energy storage and electrified manufacturing. We are particularly interested on those technologies which can meaningfully contribute to decarbonization and help avoid the worst effects of climate change. Most recently, we have studied a process invented by the Kempler Group for the electrochemical production of iron metal for steelmaking. We are also interested in low cost methods for the production of hydrogen (used for transportation and the production of fertilizer). Here are a few examples of our research on how ions interact with interfaces, how interfaces interact with electrochemical stacks, and how stacks interact with energy networks.

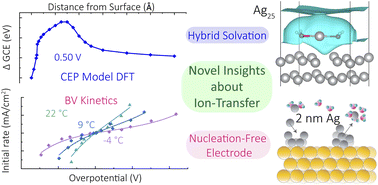
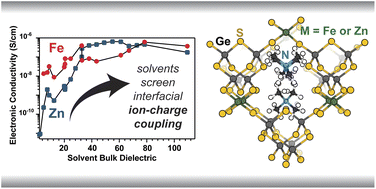
Ion transfer reactions are a fundamental step to fuel forming electrochemical reactions, electroplating for manufacturing and energy storage, and corrosion reactions that limit the durability of devices. We prepare micro- and nanoscale electrodes that act as differential reactors, allowing the direct study ion transfer kinetics. Model systems of interest include metal adatoms at single-crystal surface, dissolution and reduction at individual nanoparticles, and ion transfer across liquid-liquid interfaces.
Thurman, K., Cannan, C., Shekhar, R., Zhao, Y., Boettcher, S. W.,* Kempler, P. A.* (2025) Anions in corrosion: Influence of polymer electrolytes on the interfacial ion transfer kinetics of Cu at Au (111) surfaces. ACS Electrochemistry 1(4), 425-432
D’Antona, N., Kelly, J., Barnard, N., Ardo, S. Wang, Y., Surendranath, Y., Markland, T. E., Kempler, P. A.,* Boettcher, S. W.* (2025) Proton-Transfer Kinetics at Liquid-Liquid Interfaces. J. Amer. Chem. Soc. 147(25), 21408–21418
Shekhar, R., Mukhopadhyay, S., Sanchez, F. Konovalova, A., Boettcher, S. W., Devaraj, A., & Kempler, P. A.* (2025) Nanoporous Fe2O3 and Soluble Fe(II) Intermediates Accelerate the Electrodeposition of Fe in NaOH(aq). ACS Nano 19(37), 33449–33459 (Selected for front cover)

Research on the materials and design of electrochemical stacks is essential for realizing low-cost industrial electrochemical processes. We study advanced meshes and bipolar plates that lower stack costs, improve mass transport via controlled gas evolution, and allow continuous harvesting of metals produced via electrowinning.
Konovalova, A.,‡ Goldman, A. C.,‡ Shekhar, R., Triplett, I., Moutarlier, L. J., Kwak, M., Kempler, P. A.* (2025) Pathways to Electrochemical Ironmaking at Scale Via the Direct Reduction of Fe2O3. ACS Energy Lett. 10, 4, 1851–1857 (Selected ACS Editor’s Choice)
Noble, B. B., Konovalova, A., Moutarlier, L. J., Brogden, V. & Kempler, P. A.* (2024) Electrochemical chlor-iron process for iron production from iron oxide and salt water. Joule. 8(3) p714-727
Rajeev, M., Jerome-Saboori, A., Boettcher, S. W.,* Kempler, P. A.* (2025) Impact of dissolved iron on alkaline water electrolysis cells. ACS Catalysis 15(4), 2847–2856
Funding
We are grateful for support from the U.S. Department of Energy Basic Energy Sciences program, the Office of Energy Efficiency & Renewable Energy, Leidos, the Southern California Gas Company, and the Advanced Research Projects Agency-Energy (ARPA-E).
![]()
![]()

 Paul Kempler is an Assistant Professor in Chemistry and the Director of the Oregon Center for Electrochemistry. A Portland native, he moved to Nashville to double major in Chemical Engineering and Chemistry at Vanderbilt University under the supervision of Prof. Paul Laibinis and Prof. Kane Jennings. He completed his Ph.D. with Prof. Nathan Lewis at the California Institute of Technology in 2020 in Chemical Engineering, studying solar fuels devices converting water, sunlight, and CO2 into hydrogen and hydrocarbons. His thesis investigated
Paul Kempler is an Assistant Professor in Chemistry and the Director of the Oregon Center for Electrochemistry. A Portland native, he moved to Nashville to double major in Chemical Engineering and Chemistry at Vanderbilt University under the supervision of Prof. Paul Laibinis and Prof. Kane Jennings. He completed his Ph.D. with Prof. Nathan Lewis at the California Institute of Technology in 2020 in Chemical Engineering, studying solar fuels devices converting water, sunlight, and CO2 into hydrogen and hydrocarbons. His thesis investigated 


 Andrew Goldman graduated from Lehigh University in 2019 with a BS in Materials Science and Engineering. While at Lehigh, he worked in the lab of Professor Nicholas Strandwitz studying electrostatic phenomena at the interfaces in Metal-Insulator-Semiconductor devices for photovoltaic applications. After graduating, he started a business as a small plant nursery specializing in nut trees and other edible perennials in an effort to facilitate the transition to a more regenerative and carbon-negative food system. Now at the University of Oregon Center for Electrochemistry, Andrew is studying the relationship between process, structure, and properties of iron films electrodeposited using the Chlor-Iron process with the goal of designing full-scale reactors to decarbonize steelmaking.
Andrew Goldman graduated from Lehigh University in 2019 with a BS in Materials Science and Engineering. While at Lehigh, he worked in the lab of Professor Nicholas Strandwitz studying electrostatic phenomena at the interfaces in Metal-Insulator-Semiconductor devices for photovoltaic applications. After graduating, he started a business as a small plant nursery specializing in nut trees and other edible perennials in an effort to facilitate the transition to a more regenerative and carbon-negative food system. Now at the University of Oregon Center for Electrochemistry, Andrew is studying the relationship between process, structure, and properties of iron films electrodeposited using the Chlor-Iron process with the goal of designing full-scale reactors to decarbonize steelmaking. Stern
Stern Michaela Vacca graduated from University of California, Santa Cruz in 2024 with a B.S. in Chemistry. At UCSC, she synthesized coordination polymers for the capturing of hazardous anions in wastewater. Continuing her desire to clean up the environment, at UO she is working on the decarbonization of the steel industry while studying the formation of electrodeposited iron alloys and developing inexpensive catalysts for hydrogen evolution.
Michaela Vacca graduated from University of California, Santa Cruz in 2024 with a B.S. in Chemistry. At UCSC, she synthesized coordination polymers for the capturing of hazardous anions in wastewater. Continuing her desire to clean up the environment, at UO she is working on the decarbonization of the steel industry while studying the formation of electrodeposited iron alloys and developing inexpensive catalysts for hydrogen evolution. Ben
Ben

 Jamin Cato is a second-year undergraduate student at the University of Oregon pursuing a B.S. in chemistry. His current research work is focused on the understanding how cations influence the kinetics of the hydrogen evolution reaction in alkaline electrolytes. Jamin is interested in increasing the cultivation of renewable energy and is interested in pursuing a master’s degree upon graduation.
Jamin Cato is a second-year undergraduate student at the University of Oregon pursuing a B.S. in chemistry. His current research work is focused on the understanding how cations influence the kinetics of the hydrogen evolution reaction in alkaline electrolytes. Jamin is interested in increasing the cultivation of renewable energy and is interested in pursuing a master’s degree upon graduation.